Last month, we launched our newest service offering:
Computerised System Validation (CSV) – a practical, scalable solution designed to help MedTech companies remain audit-ready and compliant in an increasingly digital and regulated environment. But validation doesn’t stop at go-live. In fact, it’s where the real journey begins. As systems evolve and businesses adapt, so too must validation. Compliance is not static – and nor are the technologies or processes that support it.
That’s why we believe in ongoing validation:
A proactive, risk-based approach to ensure your validated systems remain compliant over time, not just on day one.
At EUMEDIQ, we’ve been asked:
“Is CSV a one-time thing?”
The answer is simple: no – and here’s why. Once implemented, validation isn’t just about initial compliance. It’s about staying compliant – even when systems evolve, regulations shift, or updates roll in unannounced.
Think of it as a living process: Software changes, team structures adapt, documentation ages – and validation must keep up.
Depending on the level of maturity in IT there are two different approaches how IT updates work. For example, if you have implemented rollout enforcement tools such as Microsoft Intune you may rely on cloud-based applications available in Microsoft App Stores. Your enforcement policy can be configured in a way that automatically new available versions will be rolled out, or only some of them, i.e. less critical, which not require re-validation for critical processes. But it is recommended that you release some applications manually before rollout enforcement and do some tests in a sandbox system as these applications might be essential for critical processes. Both options are possible within one single system.
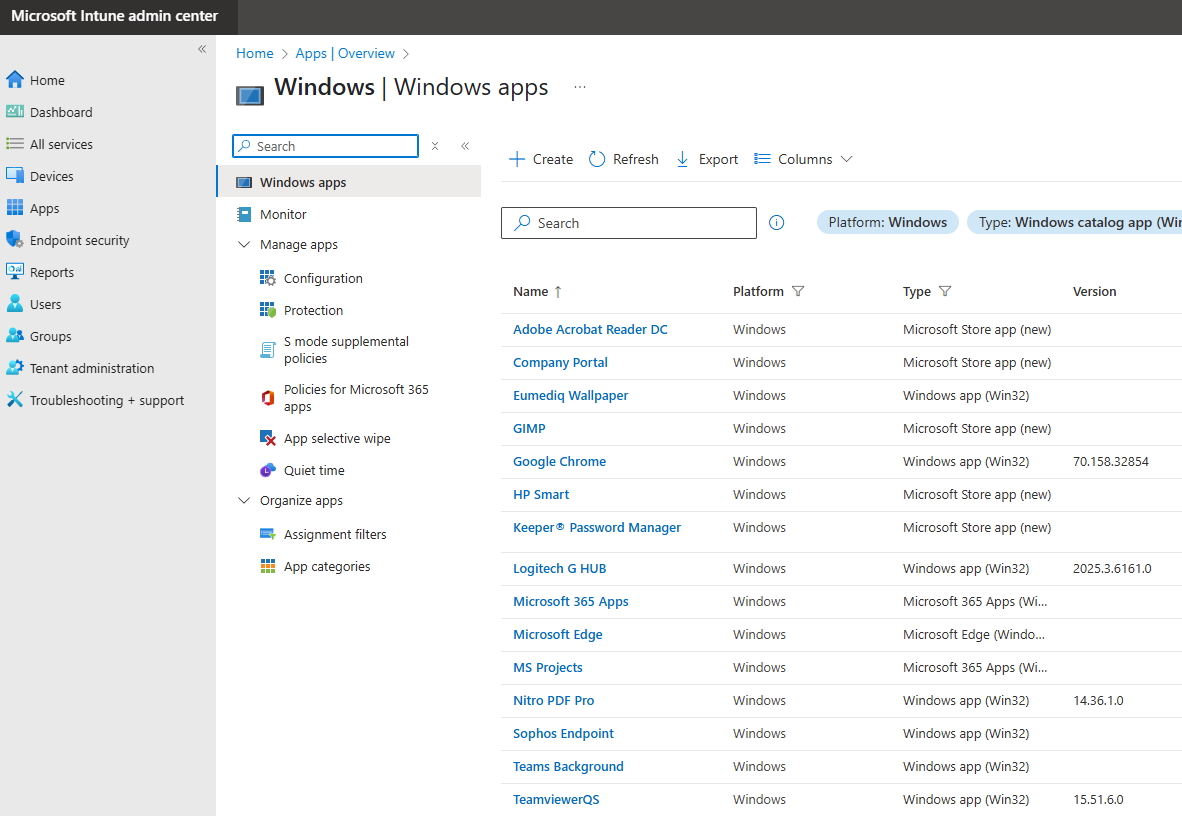
You can even manage all applications depending on the platforms, i.e. iOS, Windows, Android independent of the workstation, mobile or desktop.
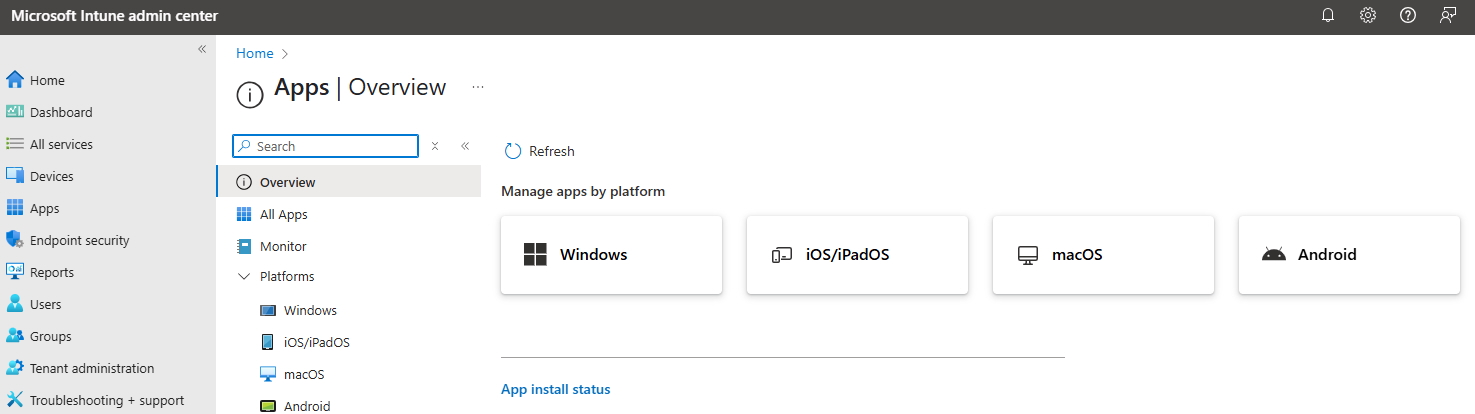
If your applications are not available via Microsoft App Store you need to provide the respective application on your own to the Microsoft Intune platform. This gives you anyway the opportunity to re-validate before “go live”, as required.
How we support ongoing validation:
✔ Monitoring changes & updates and defining enforcement policies in your IT environment
✔ Maintaining documentation status
✔ Supporting re-validation workflows
✔ Keeping your systems audit-ready
Whether you’re already working with us or just exploring the topic – our mission stays the same:
Reduce stress. Increase clarity. Keep your systems compliant.
Want to learn more about ongoing validation best practices?

