Authorised Representative

Authorised Representative
More wins with maximum efficiency
- Your qualified partner to reach your target markets
- High degree of automation and completely paperless
- Smooth and efficient registration process
- Monitoring of your importers (i.e. auditing)
- Direct collaboration in case of complaints and post-market events
- Combined expertise in regulatory, quality and auditing
- Transparent and fair cost structure
1 service provider, 3x market access
- EUMEDIQ AG based in Zug, Switzerland (headquarters)
- EUMEDIQ GmbH based in Frankfurt, Germany (EU representative services)
- EUMEDIQ Ltd. based ind London, United Kingdom (UK representative services)
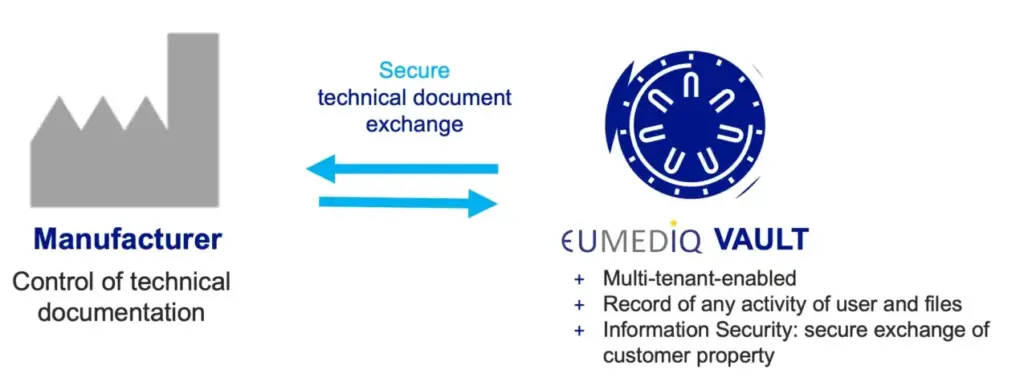
EUMEDIQ Vault
- Exclusive, ISO 27001-certified exchange platform for technical documentation
- Unique, secure document exchange and storage as a service (STaaS) solution
- One system for all EUMEDIQ supported markets
- Full access to technical documentation for customers at any time while having state-of-the-art safety standards for manufacturers
Access to your market in just a few steps
EUMEDIQ signs-off non-disclosure agreement
EUMEDIQ sends quote and service agreement templates
EUMEDIQ reviews your technical documentation
Qualification of EUMEDIQ as a supplier according to your QMS
Labelling release as Authorised Representative
Update of your EC certificate/declaration of conformity with EUMEDIQ information
EUMEDIQ sends data to competent authorities, database notifications
Frequent updates (i.e., significant changes, post-market surveillance updates)
What is an Authorised Representative?
- Service provider who takes over responsibility for medical devices and in vitro diagnostic device manufacturers and has authority to act on your behalf
- Primary point of contact for competent authorities for medical devices from third countries
- Medical device authorisation is needed:
- If legal manufacturer of medical or in vitro diagnostic devices is based abroad and wants to make products available within Switzerland, European Union or United Kingdom
- When there is no own subsidiary which can overtake the obligations
- The Authorised Representative has specific obligations, depending on national laws
Swiss Authorised Representative
- … is the primary point of contact for Swissmedic in Switzerland for products from third countries.
- … is needed if a legal manufacturer of medical devices or in vitro diagnostic devices according to MepV SR. 812.213 (MedDO) outside Switzerland wants to make products available in Switzerland and doesn’t have an own subsidiary which can overtake the obligations.
- … has specific obligations, detailed in MedDO article 52.
UK Responsible Person
- … acts on behalf of the outside-UK manufacturer to carry out specified tasks in relation to the manufacturer's obligations. This includes registering the manufacturer's devices with the MHRA before the devices can be placed on the Great Britain market.
EU Authorised Representative
- … is needed if a legal manufacturer of medical devices according to MDR 2017/745 or in vitro diagnostic devices according to IVDR 2017/746 outside the EU wants to make products available in the EU and doesn’t have an own subsidiary which can overtake the obligations.
- … has specific obligations, detailed in article 11 of both regulations.
We manage all process steps as an Authorised Representative and Importer – from the first form to market approval.
Get in touch with us and we will tailor a customised solution for your product(s).